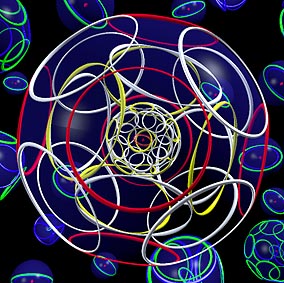
THE STRUCTURE OF DIAMOND
THE DIAMOND is a
giant molecule made only of carbon atoms bound together by covalent bonds,
in the arrangement shown at left.
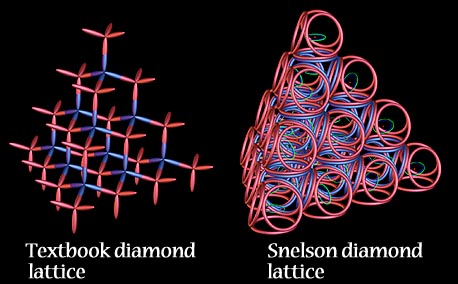
The strength of the diamond, in Snelson's model, is attributed to the matter-wave orbits' impenetrability, along with the covalent bonds. "The paradox of the diamond is interesting. Its atoms are not arranged in a tight, closest-packing order. They lack the triangulation of sound architecture. In order for its remarkable rigidity to be understood, I assume that the electrons which surround this meager structure supply it with its resistance to deformation. This is one reason why I cannot assume that electron clouds can infiltrate one another like vapors or ghosts."